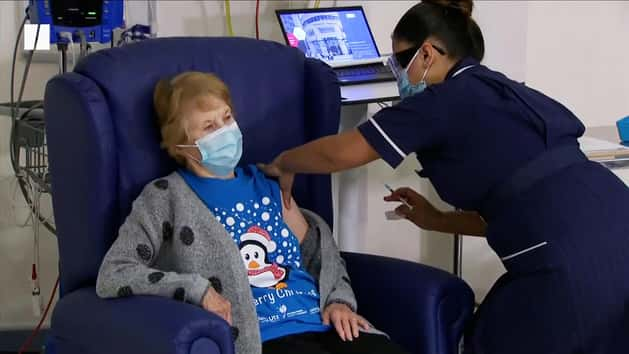
In a hugely welcome end-of-year development, the Covid-19 vaccine developed by the University of Oxford and AstraZeneca has been approved for use in the UK.
It means Britain now has two vaccines at its disposal to battle the coronavirus pandemic.
The approval was hailed on Wednesday morning as a “game-changer” by Andrew Hayward, professor of infectious diseases epidemiology at University College London.
He told BBC Breakfast: “It’s exactly what we need right now.”
When will the vaccine be available?
Health secretary Matt Hancock said the approval was “fantastic news” and confirmed that the roll out would begin on January 4.
There is considerably less uncertainty over the rollout of the Oxford vaccine, with the scene having largely been set earlier in December with the Pfizer/BioNtech jab.
The Oxford vaccine can be stored at fridge temperature for at least six months so it is hoped the logistics of administering it will be easier.
AstraZeneca said it was building up a manufacturing capacity of up to three billion doses worldwide next year, and aims to supply the UK with millions of doses in the first quarter in 2021.

Who will receive the vaccine first?
The Joint Committee on Vaccination and Immunisation (JCVI) guidance is as follows:
- Older adults in a care home and care home workers
- All those who are 80 years of age and over and health and social care workers
- All those who are 75 years of age and over
- All those who are 70 years of age and over and clinically extremely vulnerable individuals, excluding pregnant women and those under 18 years of age
- All those who are 65 years of age and over
- Individuals aged 16 to 64 years with underlying health conditions
- All those aged 60 and over
- All those aged 55 and over
- All those aged 50 and over
Not all of the people at the top of the priority list have received the Pfizer vaccine yet.
It is hoped more people in care homes will be reached with the rollout of the Oxford AstraZeneca jab due to how easy it is to store and move.
Like the Pfizer/BioNTech vaccine, people will need two doses.
But people don’t need to receive both doses of this one before protection against the virus begins.
The JCVI said data showed high efficacy from the first dose of both jabs. It has therefore advised, given the surging Covid cases in the UK, that the priority with both should be to give as many people in at-risk groups their first dose, rather than providing both doses in as short a time as possible.
Hancock has said the decision by regulators that the second dose of the Oxford vaccine can be administered up to 12 weeks after the first would speed up the rollout.
“This is important because it means that we can get the first dose into more people more quickly and they can get the protection the first dose gives you,” he told Sky News.
“The scientists and the regulators have looked at the data and found that you get what they call ‘very effective protection’ from the first dose.” This protection occurs about two weeks after the first jab, he added.
“The second dose is still important,” said Hancock, “especially for the long-term protection – but it does mean that we will be able to vaccinate more people more quickly than we previously could.”
There is another key change – while people who are pregnant and breastfeeding were previously advised against having the vaccine due to the lack of evidence, they may now take it where the potential benefits are deemed to outweigh the risks.
MHRA chief executive Dr June Raine said: “Now that we have reviewed further data that has become available, the Commission on Human Medicines has advised that the vaccine can be considered for use in pregnancy when the potential benefits outweigh the risks, following an individual discussion with every woman.”
Will I be able to choose which vaccine I get?
As things stand the vaccines will be rolled out as and when they become available.
No announcement has been made on whether one might be given priority over another as they become ready on a mass scale.
People are not expected to be able to choose which jab they want to receive.
How many doses will the UK get?
The government has secured 100m doses of the Oxford vaccine which are due to be dispatched from Germany, with a large proportion then manufactured in the UK.
This is almost enough for most of the population.
There will be four million doses available post authorisation and tens of millions of doses in the first quarter of next year, PA Media reports.
A specific schedule is difficult to establish as batches need to be quality approved by the Medicines and Healthcare products Regulatory Agency (MHRA).
Does this mean the worst of the pandemic is over?
Unfortunately not. Hancock said on Wednesday morning the approval means there is a “new route” out of the pandemic but added it would take until spring to get enough people vaccinated.
Until then, the situation facing the UK is grim. Total coronavirus cases hit a new record on Tuesday, rising above 50,000 cases for the first time, to 53,135 lab-confirmed cases.
Coronavirus patients are being treated outside some hospitals in ambulances as rising numbers put “significant pressures” on the NHS.
Footage shared on social media of Queen’s Hospital in Romford, north-east London, appears to show dozens of emergency vehicles queueing outside.
It was the latest sign hospitals in England are coming under increasing strain as the number of Covid-19 patients is at its highest ever level during the pandemic.
Hancock said: “It is going to be a difficult few weeks ahead.”
But Hancock did say he has a “very high degree of confidence” that the pandemic’s situation in the UK will change by the spring.
Asked if he could provide a timeline for when under-50s without pre-existing conditions may be vaccinated, Hancock told Times Radio: “It depends on the speed of manufacture, I wish I could give you a date, your invitation right now, but we can’t because it depends on the speed of the manufacture.
“This product, it’s not a chemical compound it’s a biological product so it’s challenging to make, so that is the rate-limiting factor in terms of the rollout.
“Now that we have two vaccines being delivered we can accelerate, how fast we can accelerate will be determined by how fast the manufacturers can produce.
“But what I can tell you is that I now have a very high degree of confidence that by the spring enough of those who are vulnerable will be protected to allow us to get out of this pandemic situation.”

How does it compare to other vaccines?
Oxford data indicates the vaccine has 62.1% efficacy when one full dose is given followed by another full dose, but when people were given a half dose followed by a full dose at least a month later, its efficacy rose to 90%.
The combined analysis from both dosing regimes resulted in an average efficacy of 70.4%.
As well as the Pfizer vaccine which has an efficacy of 95%, there are a number of other jabs the UK has secured doses of.
Final results from the trials of Moderna’s vaccine suggest it has 94.1% efficacy, and 100% efficacy against severe Covid-19.
The early contenders all have high efficacy rates, but researchers say it is difficult to make direct comparisons because it is not yet known exactly what everyone is measuring in the trials.
What about cost?
Pfizer/BioNTech is making its vaccine available not-for-profit.
According to reports, the Moderna vaccine could cost about $38 (£28) per dose and the Pfizer candidate could cost around $20 (£15).
Researchers suggest the Oxford vaccine could be relatively cheap to produce, with some reports indicating it could be about £3 a dose.
AstraZeneca said it will not sell it for a profit, so it can be available to all countries.
However, the details of the deals made by the UK government have not been made public.